
It has been shown that the activity of both HA synthases and Hyals can be regulated by cytokines and growth factors ( 30, 31). PH20 expression seems to be limited to testes, but its mRNA can be detected in other tissues and in malignancies ( 28, 29). Among them, Hyals 1, 2, and 3 are widely expressed in somatic tissue and in cancer ( 26, 27). Six hyaluronidase genes have been reported in humans: Hyal1, Hyal2, and Hyal3, clustered on chromosome 3p21.3, and Hyal4, PH20 and PHyal1, clustered on chromosome 7q31.3 ( 26). In contrast, the role of Hyals in HA degradation at the airway surface and subepithelial tissues has not been previously explored.


Hyaluronidase vs vtrace skin#
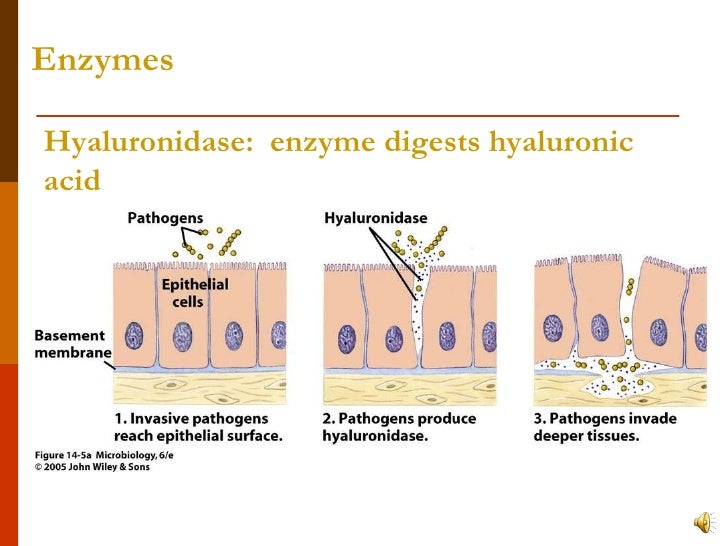
ROS induce HA depolymerization in a variety of tissues, including skin ( 22), cartilage ( 23), and at the airway surface ( 21, 24, 25). Hyaluronan fragments can be generated by reactive oxygen species (ROS)-induced depolymerization and/or by the activity of the hyaluronan degrading enzymes, the hyaluronidases (Hyals). We have previously reported that high-molecular-weight HA found at the surface of airway epithelium is involved in airway homeostatic mechanisms by binding and regulating the activity of enzymes such as lactoperoxidase and tissue kallikrein ( 18, 19), while small HA fragments stimulate ciliary beating through a mechanism that involves the receptor for hyaluronic acid–mediated motility (RHAMM) ( 20, 21). Most HA functions have been shown to be size-dependent: the high-molecular-weight molecules have been reported to exert anti-inflammatory and immunosuppressive effects ( 16), while smaller fragments stimulate gene expression and protein synthesis of pro-inflammatory mediators such as cytokines and chemokines (for review see Ref. In the lungs, HA is involved in injury and repair processes through its receptor CD44 ( 12), in interstitial fluid balance regulation due to its high water-binding capacity ( 13), and in the induction of inflammatory mediators in alveolar macrophages and lung fibroblasts ( 14, 15). Although hyaluronan was discovered in 1934 ( 7) and its structure defined in 1951 ( 8), only recently it has become clear that, besides its structural role as a component of the extracellular matrix (ECM) ( 9), HA is involved in a broad range of biological processes associated with health and disease (for review see Refs. In the airways, HA is produced by submucosal glands and by superficial airway epithelial cells ( 3, 4), where it is synthesized by hyaluronan synthases (HAS) at the plasma membrane and released as a high-molecular-weight polymer into the extracellular space ( 5, 6). Hyaluronan (HA) is a glycosaminoglycan widely distributed in tissues and is a normal constituent of airway secretions ( 1, 2). This article addresses mechanisms of hyaluronan degradation in human airways associated with inflammatory responses. Our observations indicate that Hyal 1, 2, and 3 are expressed in airway epithelium and may operate in a coordinated fashion to depolymerize HA during inflammation associated with up-regulation of TNF-α and IL-1β, such as allergen-induced asthmatic responses. In addition, increased expression and activity were observed in tracheal sections and in bronchoalveolar lavage (BAL) obtained from subjects with asthma when compared with normal lung donors and healthy volunteers. Tissue sections from normal individuals and from individuals with asthma showed a Hyal distribution pattern similar to that observed on nontreated HBE cells or exposed to cytokines, respectively. Confocal microscopy showed that Hyals 1, 2, and 3 were localized intracellularly, while Hyal2 was also expressed at the apical pole associated with the plasma membrane, and in a soluble form on the apical secretions. We found that Hyal-like activity is present in the apical and basolateral secretions from HBE cells where Hyals 1, 2, and 3 are expressed, and that IL-1β acts synergistically with TNF-α to increase gene expression and activity. Because TNF-α and IL-1β induce Hyals in other cells, we tested their effects on Hyals expression and activity. Since HA depolymerization initiates a cascade of events that results in kinin generation and growth factor processing, in the present work we used primary cultures of human bronchial epithelial (HBE) cells grown at the air–liquid interface (ALI) to assess hyaluronidase (Hyal) activity by HA zymography, gene expression by quantitative real-time PCR, and localization by confocal microscopy. Hyaluronan (HA) is present at the apical surface of airway epithelium as a high-molecular-weight polymer.


 0 kommentar(er)
0 kommentar(er)
